To Patent or Publish—That Is the Question
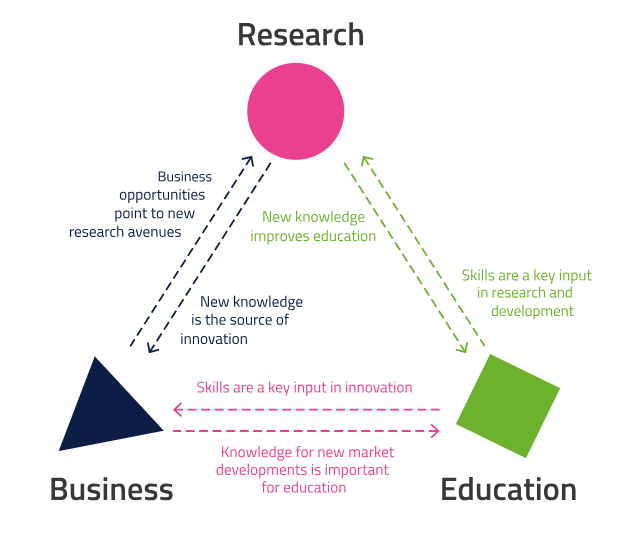
Introduction In the world of science and innovation, a key dilemma often arises: is it better to file an invention for patent protection, or to publish the findings right away? Both paths have their pros and cons, and the right choice depends on many factors. When to file for patent protection? 📅 Always before public …